
Technology
Genelux aims to provide an elegant therapeutic approach. Our oncolytic immunotherapy drug candidates are “off-the-shelf” personalized therapeutics. In other words, while we administer the same virus product to different patients, the cellular immune response generated is specific to the unique neoantigens in that patient. We believe that our approach may offer significant advantages over other approaches to anticancer treatments.
Oncolytic Immunotherapy
Oncolytic immunotherapy is the treatment of cancer with viruses that selectively replicate in tumors, but not in normal tissues, which kills cancer cells in two ways. First, oncolytic viruses replicate in the tumor cell until the cell “bursts”, resulting in the lysis of tumor cells. Second, when the cancer cells die, they release tumor neoantigens, which are taken up by cells of the immune system and alert the body’s T-cells to search and destroy other cancer cells in the body. This oncolytic process establishes long-term antitumor immunity to unleash the full potential of the body’s immune system and improve outcomes for cancer patients.
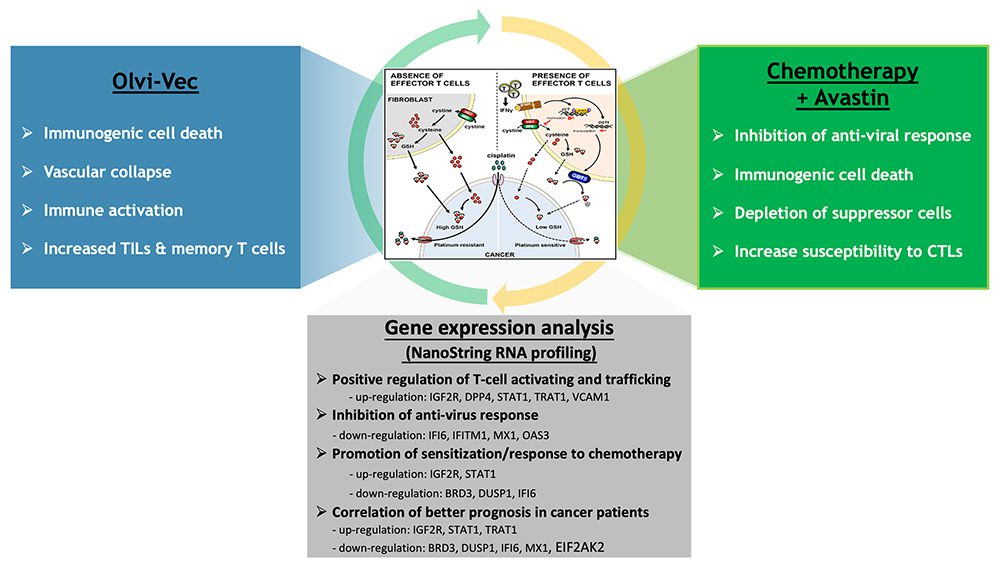
Modulating the Tumor Microenvironment: Overcoming Chemoresistance
Olvi-Vec-primed immunochemotherapy delivers synergistic anti-tumor activities through multifaceted and complimentary mechanisms of action.
The ChoiceTM Discovery Platform
Our proprietary ChoiceTM discovery platform is the foundation of our oncolytic immunotherapy product development program and is designed to allow us to generate new product candidates rapidly from conception through the initiation of clinical trials.
Comprehensive Approach: ChoiceTM is powerful and modular and has been used to develop an extensive library of engineered and selected strains of the oncolytic vaccinia virus identified from multiple in vitro (e.g., viral replication rate, plaque size, transgene expression efficiency, etc.) and in vivo (e.g., viral titer, antitumor activities, safety, etc.) selection criteria.
Highly Productive: ChoiceTM has produced more than 500 different versions of the vaccinia virus armed with greater than 110 transgenes, having a variety of engineered attributes, including immune modulatory and cell killing properties.
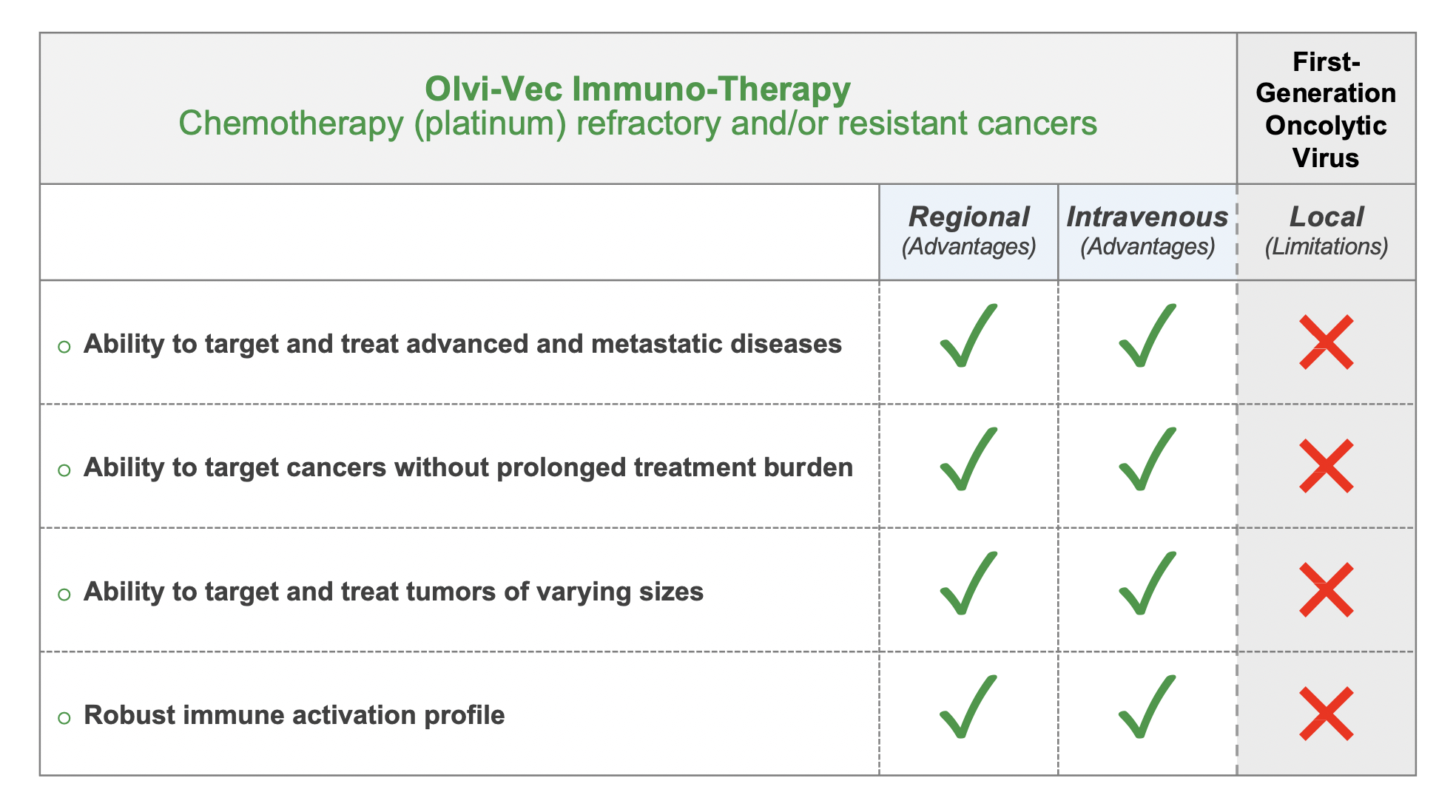
Unmeasured Potential: These provide potential utility in multiple tumor types in both monotherapy and combination therapy settings, via physician-preferred routes of administration techniques, including regional (e.g., intraperitoneal) and systemic (e.g., intravenous) delivery routes.

ChoiceTM Library
We have generated an extensive portfolio of oncolytic vaccinia immunotherapy clinical candidates, having a variety of engineered attributes, including the immune modulatory and cell killing payloads set forth below:

-
- Immune Modularity Molecules
- Single-Chain Antibodies
- Anti-Angiogenic Genes
- Cell Growth & Differentiation Regulators
- Metastasis Suppressor Genes
- Clonal Isolated Strains (non-GMO)
- Cell Matrix-Degradative Genes
- Apoptosis Inducing Genes
Our library of over 500 different oncolytic vaccinia constructs and strains armed with greater than 110 transgenes have demonstrated the ability to cause:
-
- Regression and elimination of a wide range of tumor types (pre-clinical signals of efficacy against 20 tumor types)
We intend to develop one or more therapies derived from this platform to address multiple types of tumors.
Lead Product Candidate: Olvi-Vec (olvimulogene nanivacirepvec), our lead product candidate, was selected from our library based on the ability to optimize the natural multi-modal properties of the Lister strain of vaccinia virus (the Olvi-Vec “backbone”).



